
RESEARCH PROJECTS
iPSC-derived CARDIAC ORGANOID
Induced pluripotent stem cells (iPSCs) are reprogrammed adult cells that can generate functional human tissue, making them invaluable for patient-specific disease modeling. Moving beyond simple 2D cultures, researchers create self-assembled cardiac organoids—miniature heart tissues scaled to approximately 3mm in size. These organoids are designed to fully recapitulate the complexity of the native heart tube. Through spontaneous organization, they develop a 3D chamber structure and a true multicellular lineage, containing not only heart muscle cells (cardiomyocytes) but also vascular endothelial cells, fibroblasts, and immune cell populations. This highly organized architecture provides a powerful, physiologically relevant platform for studying complex congenital heart defects.
DESIGN OF A CONTRACTILE AUXETIC CARDIAC PATCH
Typically, cardiac patches are designed with material properties to match native cardiac tissue and provide a scaffold for repair and regeneration. However, the material design rarely accounts for ventricle anisotropy and ranges in mechanical properties. A cardiac patch with the ability to account for this behavior could be used to improve cardiac function post myocardial infarction. In collaboration with the Dr. Hollister lab at Georgia Tech, we are designing an auxetic cardiac patch using hiPSC-derived cardiomyocytes. The auxetic scaffold allows for favorable anisotropy, tunable strength, and improved shear resistance. We hope to investigate cardiomyocyte maturation and promote favorable remodeling of the infarcted region.
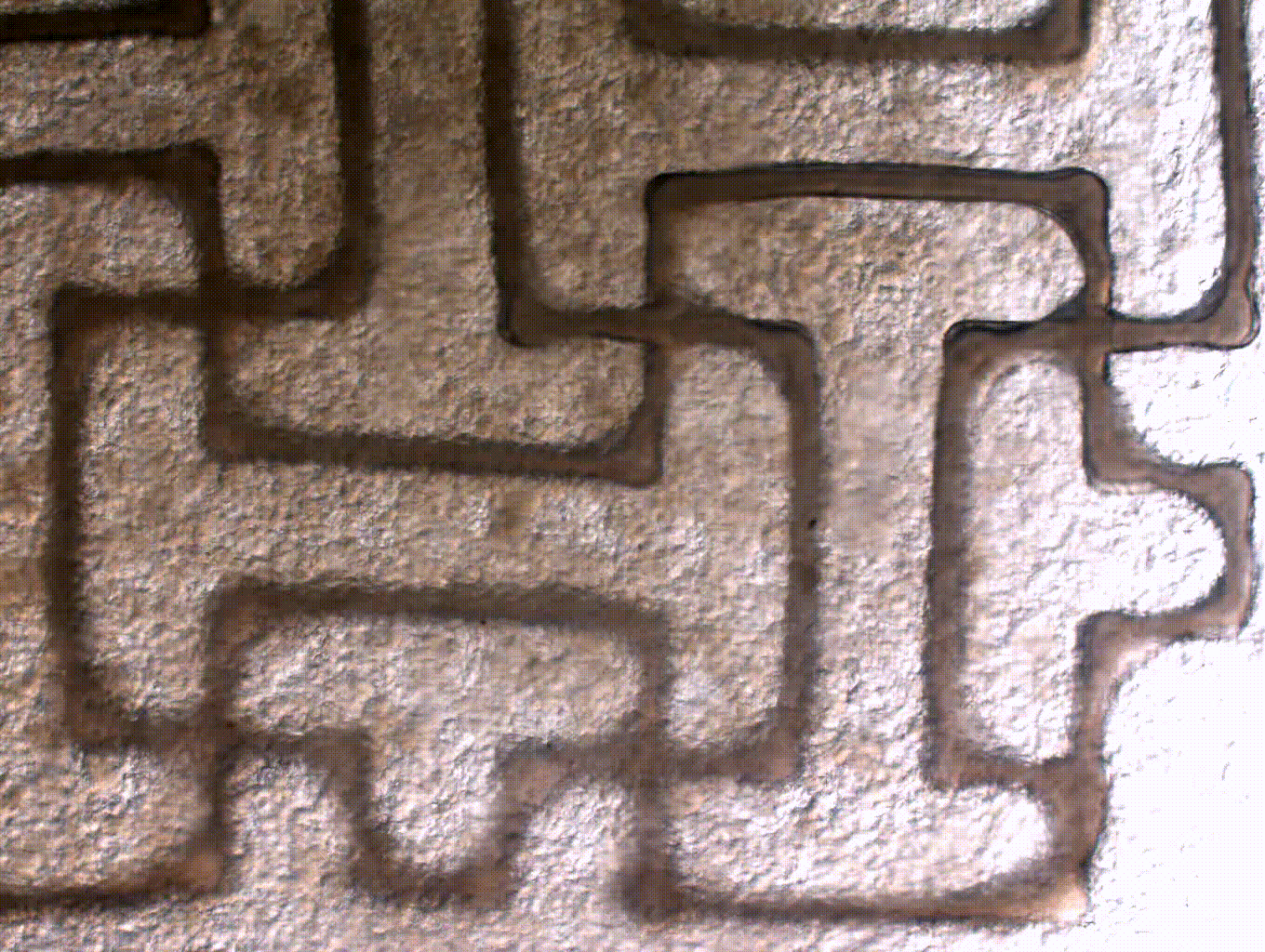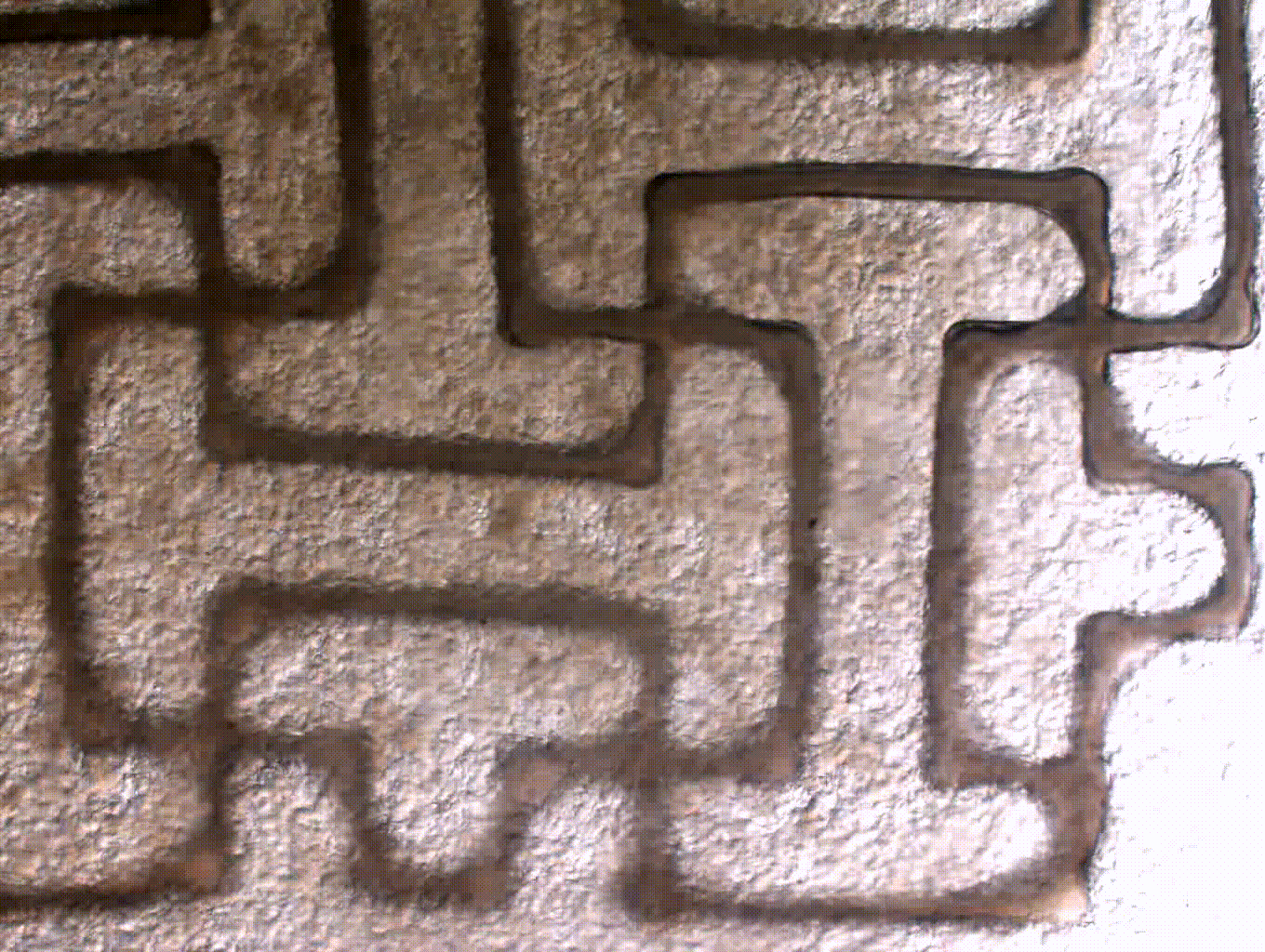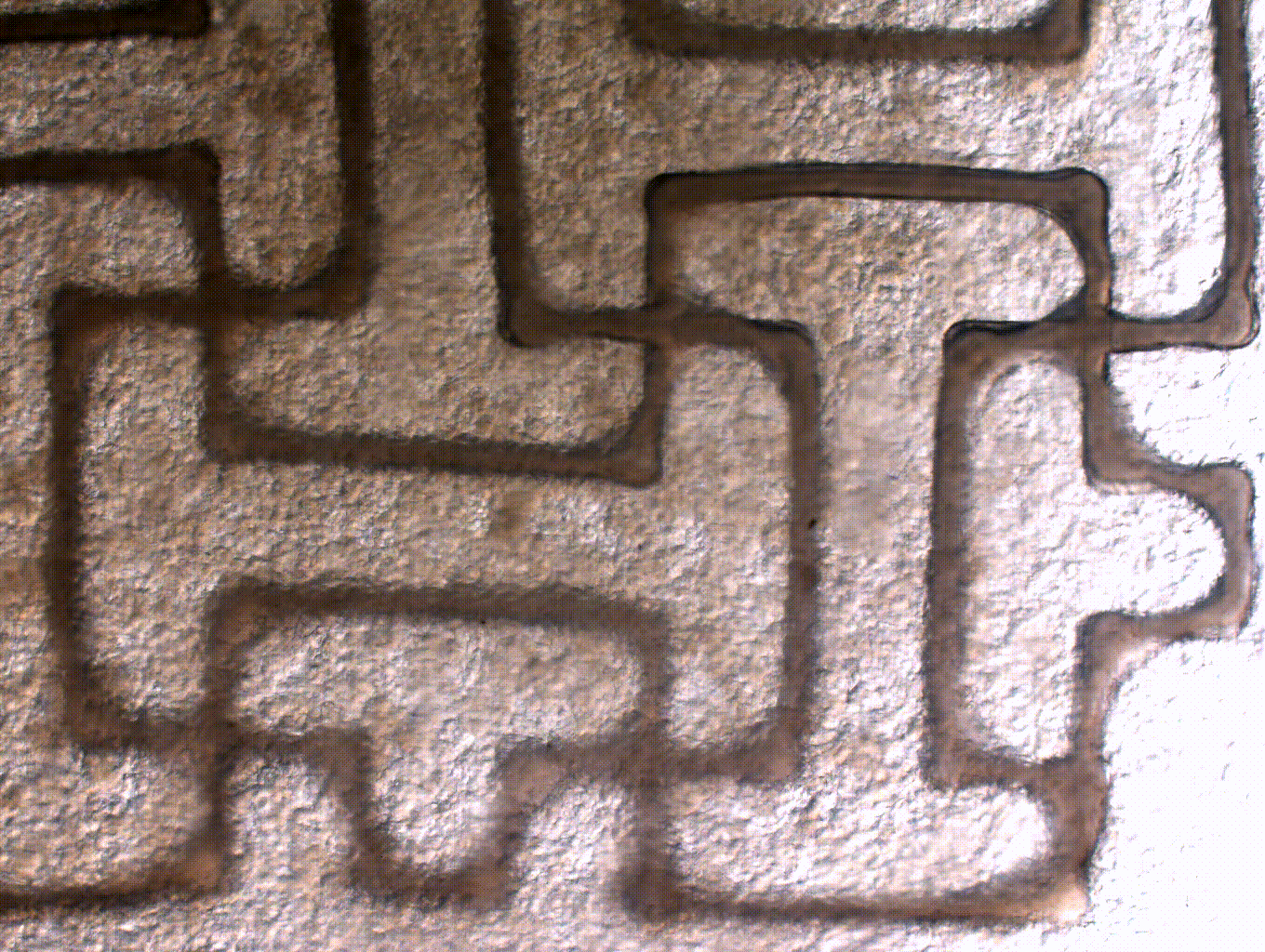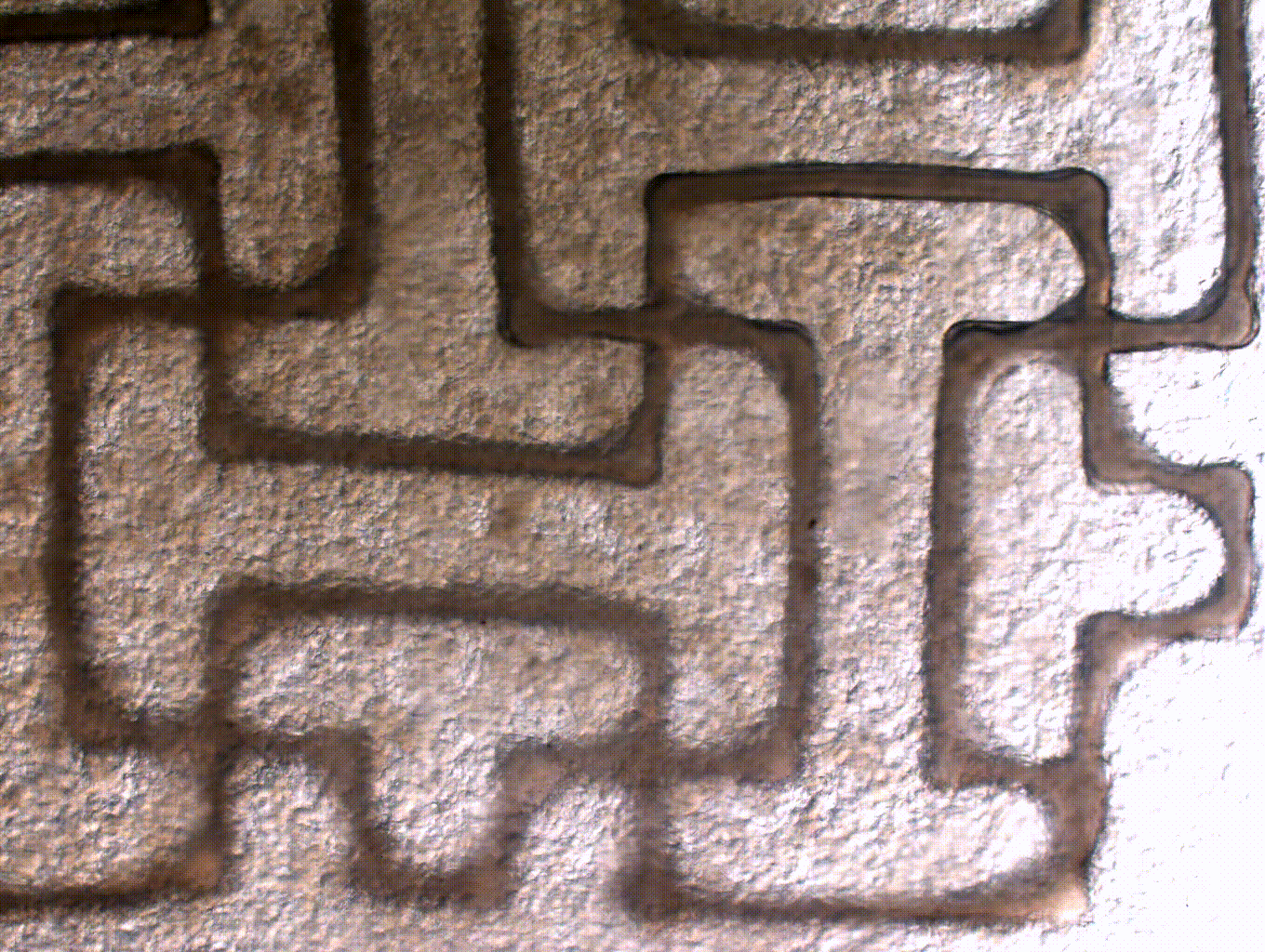
ENGINEERING A FUNCTIONAL AORTIC HEART VALVE USING A 3D BIOPRINTING APPROACH
Aortic valve disease is one of the most common congenital heart defects and affects over 5% of children with heart disease. Often times, pediatric patients are treated by replacing or less commonly repairing the valve. The issue with clinically available valve implants for children is that they come at a fixed size and are incapable of growth. Thus, this requires multiple surgeries for valve revisions. Our goal is to address this need with a tissue engineered heart valve that is capable of remodeling and growth within a growing child. To do so, we are using induced pluripotent stem cells to generate patient specific valve cells. In addition, we are engineering cell-laden biomaterials to be 3D printed using 3D models generated from patient CT. Using this approach, we will generate a 3D tissue engineered heart valve that has the potential to grow and repair in a child.

ENGINEERING EXOSOME-LIKE VESICLES
Stem cell based cardiac regeneration occurs mainly through paracrine signaling to local tissues. A key component of the paracrine signals released by the stem cells are nano-sized vesicles called exosomes. These are vehicles used to transfer microRNA, proteins and lipids to the native tissue. The therapeutic effects of such signaling persists long after the implanted stem cells have been flushed out, highlighting their importance in regenerative therapies. We want to artificially engineer exosomes in-vitro so we can modulate the type and concentration of cargo delivered and also target specific tissues based on membrane proteins we attach. This will provide a highly controllable and specific avenue of paracrine signaling for regenerative therapies.
EXOSOMAL RNA CONTENT MANIPULATION FOR ENHANCED MYOCARDIAL REPAIR
Despite recent advances in cell therapy, donor-to-donor variability challenges its therapeutic potential. With bioinformatics approaches, it is possible to identify and predict essential molecules such as miRNA, mRNA, and lncRNA that are important in cardiac repair. In this project, we are deciphering specific RNAs' role and mode of action on cardiac repair in an ischemia-reperfusion model of MI. Specific miRNA, identified from previous bioinformatics studies, are inhibited or overexpressed in CPC exosomes to enhance therapeutic potential in various cardiac diseases.

BACTERIAL NANOCELLULOSE-FIBRIN BIOINK FOR ALIGNED CARDIAC TISSUE

Myocardial infarction results in irreversible loss of cardiomyocytes and structural weakening that the adult heart cannot regenerate, creating a need for engineered tissues capable of restoring mechanical integrity and contractile function. This work addresses this challenge through the development of 3D-bioprinted cardiac constructs that replicate the anisotropy, mechanical behavior, and micro-architecture of native myocardium. A fibrin–bacterial nanocellulose (BNC) composite bioink is formulated and optimized for printability, structural fidelity, and myocardial-like mechanical properties. Using advanced 3D bioprinting strategies, scaffolds with controlled fiber alignment are fabricated to support organized cardiomyocyte attachment, maturation, and function. Human iPSC-derived cardiomyocytes are seeded onto these constructs to evaluate viability, alignment, synchronous beating, and mechanical coupling. This approach advances the development of engineered cardiac patches designed to enhance myocardial repair and functional recovery following injury.

SINGLE CELL RNA SEQUENCING TO IDENTIFY REGENERATIVE CELL SUBPOPULATIONS
Regenerative therapies utilizing ckit+ cardiac progenitor cells for repairing the myocardium exhibit age-dependent capabilities, with older patient cells inducing increased fibrotic and immunogenic responses. We are utilizing the high-resolution capabilities of single cell RNA sequencing to analyze cells collected from neonates, children, and adults to identify subpopulations driving these differences in outcomes. We have identified subpopulations of cells in neonate and adult populations corresponding to proliferation, wound healing, and immunogenic activity. Ultimately, we are seeking a way to separate more regenerative cell types from patient samples to improve therapeutic outcomes.
USING SYSTEMS BIOLOGY AND BIOINFORMATICS TO IDENTIFY POTENITAL THERAPEUTIC RNA CLUSTERS IN HUMAN CARDIAC C-KIT+ CELLS
The regenerative potential of human cardiac c-kit+ progenitor cells (hCPCs) to repair myocardium after injury has been shown in multiple studies. In this project, we sequence RNA from a large pool of patient-derived hCPCs for miRNAs of interest. Bioinformatics and systems biology-based approaches will be used to (1) identify miRNA clusters which covary with functional, pro-reparative outcomes, and (2) identify potential targets of interest for further study. We aim to gain insight into the molecular mechanisms by which hCPCs regenerate cardiac tissue.

